Abstract
In vitro erythroid cell generation represents an important model and potential therapeutic strategy for hemoglobin disorders, including sickle cell disease. Traditional methods for erythroid cell generation from human embryonic stem cells (hESCs) via embryoid body or monolayer culture methods result in mainly ε-globin and γ-globin production with very low amounts of β-globin. Recently, we have demonstrated hESC-derived erythroid cell generation via hemangioblast-like ES-sacs, allowing for definitive β-globin production at detectable protein levels by HPLC (Stem Cells, 2016).
In this study, we sought to improve ES-sac generation using defined serum-free Knockout Serum Replacement (KSR) instead of fetal bovine serum (FBS), to allow for more precise optimization and variability reduction among FBS lots. We investigated ES-sac generation using VEGF by comparing 15%FBS-based media (Control) and serum-free 20%KSR-based media (MEF-KSR) on C3H feeder cells using H1 hESCs (maintained on MEF feeder cells). In addition, we compared feeder-free hESCs (maintained on Matrigel) with on-feeder hESCs using both KSR-based media (MT-KSR) and FBS-based media (MT-FBS) to determine whether feeder-free hESCs culture produces a more uniform cell population. 15 days after ES-sac generation, hematopoietic-like suspension cells were differentiated to erythroid cells with erythropoietin for an additional 15 days (total 30 days). After ES-sac generation (day 15), we counted ES-sac numbers (from 1x10e5 hESCs), and analyzed definitive hematopoietic precursor cells (HPCs, CD34+CD45+), erythromegakaryocyte precursors (EMPs, CD235a+CD41a+), and primitive erythrocytes (PEs; CD235a+CD34-) by flow cytometry. After erythroid differentiation (day 30), we evaluated erythroid cell yields (from 1x10e5 hESCs) and globin protein levels by HPLC.
We observed similar amounts of ES-sacs (62±48 vs 40±30, ns) at day 15 and erythroid cell yields (32±19 vs 41±12 x10e5, ns) at day 30 as well as similar levels of β-globin (13±10 vs 19±9%, ns) and ε-globin (21±10 vs 12±4%, ns) between MEF-KSR and Control. The MEF-KSR results in similar percentages of HPCs (4±2 vs 7±5%, ns), EMPs (9±6 vs 14±14%, ns), and PEs (25±11 vs 36±13%, ns) in ES-sacs, suggesting that KSR can replace FBS allowing for similar ES-sac generation with erythroid differentiation derived from on-feeder hESCs.
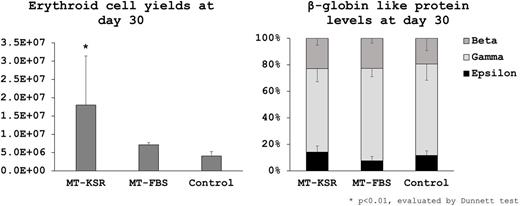
When using feeder-free hESCs, ~10-fold greater amounts of ES-sacs (564±458 and 208±138 vs 40±30) and erythroid cell yields (180±134, p<0.01 and 71±6 vs 41±12 x10e5) were obtained in MT-KSR (p<0.01) but not MT-FBS (ns), compared to Control (Figure). Lower percentages of HPCs (2±2, p<0.01 and 6±4, ns vs 7±5%), EMPs (7±4 and 7±2 vs 14±14%, ns), and PEs (22±10 and 20±3 vs 36±13%, p<0.05) were observed in MT-KSR and MT-FBS, while high levels of β-globin (23±5 and 23±4 vs 19±9%, ns) and low levels of ε-globin (14±5 and 8±3 vs 12±4%, ns) were detected at similar protein levels among all groups (Figure). These data demonstrate that our serum-free culture (MT-KSR) allows for greater amounts of ES-sac generation, resulting in more erythroid cell yields with high β-globin production.
To evaluate whether KSR-based media improve ES-sac colony formation in the initial step or ES-sac structure production in the maturation step, we investigated either KSR- or FBS-based media in the first half and second half of ES-sac generation from feeder-free hESCs: switched from KSR to FBS (KSR-FBS) or FBS to KSR (FBS-KSR), as compared to Control (FBS-based media only). We observed a trend to higher amounts of ES-sac generation (157±74 and 88±16 vs 48±20, ns), lower percentage of HPCs (1±1, p<0.05 and 6±1, ns vs 4±2%), higher percentage of EMPs (10±2, p<0.05 and 1±1, ns vs 5±1%), and higher percentage of PEs (27±7, p<0.01 and 8±2, ns vs 16±1%,) were observed in KSR-FBS (similar to KSR only) but not in FBS-KSR (similar to FBS only). These data suggest that KSR-based media allows for improved ES-sac colony formation in the initial step, while FBS may increase emergence of definitive hematopoietic precursors during ES-sac generation.
In summary, we demonstrate improved ES-sac colony formation with serum-free culture from feeder-free hESCs producing erythroid cell yields with high β-globin production (up to 23%) detectable at the protein level. Further improvement of hESC-derived erythroid cell generation might be achieved by additional nutrients or cytokines in defined KSR-based media.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal